In the first part, we were going to measure 3 different metals' length, diameter, and mass in order to get the density with its uncertainty.
And then, we get:
metal length diameter mass density
- steel 5.00+-0.01 cm 1.26+-0.01 cm 48.9+=0.1 g 7.6 g/cm^3
- copper 5.14+-0.01 cm 1.28+-0.01 cm 58.4+=0.1 g 8.8 g/cm^3
- brass 4.80+-0.01 cm 1.60+-0.01 cm 80.0+=0.1 g 8.3 g/cm^3
From the equation for density:
And then, we take the natural logarithm of each side we get:
=>
- steel
- copper
- brass
- steel: 7.6+-0.15 g/cm^3
- copper: 8.8+-0.16 g/cm^3
- brass: 8.3+-0.12 g/cm^3
And, there is next lab to determine the mass of object by measuring two angles and those forces of two strings.
All of first, we focus on the y size of the object.
and then, we focus on the x size of the object.
The data we measure about force on strings and angles:
F1=7.5+-0.5N
F2=7.0+-0.5N
a1=46degree+-1degree
a2=50degree+-1degree
plug those data to equation.
And we can get the force from equation
is 1.0976+-0.015N


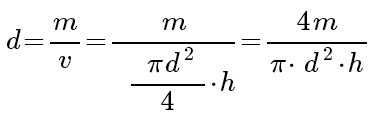





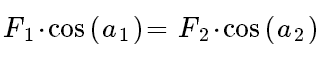

没有评论:
发表评论